Abstract
In this study, we performed an adipogenic differentiation of human adipose-derived stem cells (ADSCs) in vitro with different deuterium content (natural, low and high) in the culture medium during differentiation process with parallel analysis of the gene expression, metabolic activity and cell viability/toxicity. After ADSCs differentiation into adipocytes we have done the analysis of differentiation process efficiency and determined a type of resulting adipocytes (by morphology, gene expression, UCP1 protein detection and adipokine production analysis). We have found that high (5 × 105 ppm) deuterium content significantly inhibit in vitro adipogenic differentiation of human ADSCs compared to the groups with natural (150 ppm) and low (30 ppm) deuterium content. Importantly, protocol of differentiation used in our study leads to white adipocytes development in groups with natural (control) and high deuterium content, whereas deuterium-depleted differentiation medium leads to brown-like (beige) adipocytes formation. We have also remarked the direct impact of deuterium on the cellular survival and metabolic activity. Interesting, in deuterium depleted-medium, the cells had normal survival rate and high metabolic activity, whereas the inhibitory effect of deuterated medium on ADSCs differentiation at least was partly associated with deuterium cytotoxicity and inhibitory effect on metabolic activity. The inhibitory effect of deuterium on metabolic activity and the subsequent decrease in the effectiveness of adipogenic differentiation is probably associated with mitochondrial dysfunction. Thus, deuterium could be considered as an element that affects the substance chirality. These findings may be the basis for the development of new approaches in the treatment of obesity, metabolic syndrome and diabetes through the regulation of adipose-derived stem cell differentiation and adipocyte functions.
Introduction
In the 21st century, non-communicable diseases (NCD) like obesity, metabolic syndrome and type 2 diabetes mellitus (T2DM) became the main medical problems of the humanity1,2,3,4. These diseases started in the Western world, but in parallel with the improving of human life standards, technological progress and the spread of the Western lifestyle also around the world. So, these diseases have become a global epidemic5. Currently, although there remains a correlation between the level of economic development and the frequency of these diseases, they have ceased to be a medical problem in high-income countries, but also have become an urgent item for the low-income and middle-income countries6.
A characteristic feature of obesity, metabolic syndrome and T2DM is the defection of glucose and lipids metabolism, which is manifested in insulin resistance, impaired fasting glucose, dyslipidemia, high blood sugar, high serum triglycerides, imbalance of different types of lipoproteins in blood serum7,8.
Prevention and treatment of these diseases include changing and controlling lifestyle, diet and the use of pharmaceuticals9. Despite the progress in medical science, pharmacology (the development of new substances for the correction of metabolism) and biotechnology (improving the process of insulin production), the solution to the problem of obesity, metabolic syndrome and T2DM requires new effective approaches.
It should be taken into account that adipose tissue is one of the key players in the development of obesity, metabolic syndrome and T2DM10. Conversely, adipose tissue can also be considered as the main target for the prevention and treatment of these pathological conditions11. The adipose tissue in the human body can be classified by anatomical location (subcutaneous, visceral, intermuscular, yellow bone marrow and breast), as well as functions (white and brown fat)12. The main function of white adipose tissue is to preserve energy in the form of lipids, insulating organs, and endocrine function - the production of hormones, growth factors, cytokines, chemokines and other biologically active substances that regulate energy metabolism and many other body functions, which were called adipokines13. The function of brown adipose tissue is heat production during adaptive thermogenesis. In humans, unlike rodents (laboratory animals most widely used in medical experiments, including modeling of obesity, metabolic syndrome and T2DM), brown adipose tissue is present in significant amount only in newborns and infants14. Recently, the existence of active thermogenic adipose tissue in human adults was shown, but this adipose tissue differs from the classical brown adipose tissue in a number of aspects (development, morphology, gene expression, adipokine production etc)15. This adipose tissue is called “beige” or “brite” (brown in white) adipose tissue. All types of adipocytes arise from adipose-derived stem cells (ADSCs) in the process of differentiation. At present, a number of questions regarding the origin of beige adipocytes (from the same stem cell as white adipocytes, or from the same stem cell as brown adipocytes, or from own stem cell) remain debatable as well as the ability of white adipose tissue to transdifferentiate into brown/beige adipose tissue16.
The ability to control the formation of new adipose tissue, to convert white adipose tissue into brown/beige adipose tissue, or to set the direction of differentiation of ADSCs into a specific subtype of adipocytes is an attractive target for the development of new pharmacological substances to treat obesity, metabolic syndrome and T2DM17.
In addition to the search for new pharmacological substances targeted to adipose tissue functions and/or various other biochemical aspects of energy homeostasis, it is also important to study the role of water in human health, metabolism, and pathogenesis of different diseases. Water is the most common chemical substance on the Earth and constitute the largest mass part of living organisms in percentage ratio. Water is also a universal solvent in which the basic biochemical processes of living organisms occur. An important component of a healthy diet is consumption of drinking water instead of sugar-containing and carbonated beverages18,19. So, the modulation of biological and physicochemical properties of water is also a promising opportunity to improve the effectiveness of treatment for obesity, metabolic syndrome and T2DM.
Deuterated water has the same chemical formula as ordinary water, but instead of two atoms of a light hydrogen isotope (protium) it contains two atoms of a heavy hydrogen isotope (deuterium). Deuterated water shows only slight cytotoxicity. In general, chemical reactions in deuterated water have slower rate than in ordinary water20,21,22,23.
Deuterium can be considered as a regulator of the biological properties of normal and/or cancer cells24,25,26,27. One of the medicine trends is the development of deuterium-containing drugs28,29,30. The other direction refers to the role of isotopology D/H ratio and its change in water to be used as an adjuvant in cancer treatment31,32,33,34. Different D/H ratio is manifested in the form of kinetic isotope effect, which is characterized by a change in the biotransformation and excretion rates of the drugs35,36,37. Moreover, the methodological approaches for drugs quality control based on water isotopology, could reduce the toxic load of the body38,39.
In our previous studies, we have shown that different deuterium concentration in growth medium affects the proliferation, migration and metabolic activity of cultured human adipose-derived stem cells (ADSCs)40.
In present study, we consider the question whether deuterium is involved in regulation of ADSCs differentiation, different types of adipocytes development and an adipokine production by adipocytes. Adipogenic differentiation of ADSCs was chosen as a model for our in vitro pilot study, where we compared the efficiency of adipogenesis in media with different deuterium contents (natural, low and high).
Materials and Methods
Types of water used in the study. Water preparing and testing (characterization)
The following basic water samples with various deuterium content were used in this study: deuterium-depleted water (ddw) with D/H = 4 ± 2 ppm D (Sigma-Aldrich, USA); deuterated water (D2O) with D/H = 99 absolute at. % D (Sigma-Aldrich, USA). Growth media with various deuterium content were prepared by diluting deuterium-depleted and deuterated water. The following media for adipogenic differentiation were used in this study: № 1 - medium with D/H ratio 30 ppm; № 2 - the medium with the highest deuterium content - D/H ratio 500.000 ppm. The milliQ water (MilliQ-system, UK) served as a standard (control) with D/H ratio 150 ppm. MilliQ, deuterium-depleted and deuterated water had no differences in physical characteristics or in trace element composition, except the deuterium content. This excluded the multifactor influence in the system for all comparison groups. Detailed description of the method was presented in previous studies22,27,40,41.
The deuterium content was controlled by multipass laser absorption spectroscopy on the Isotopic Water Analyzer-912-0032 (Los Gatos Research Inc., USA). Detailed description of this method was presented in previous studies22,27.
Chemical analysis of water with various deuterium content was performed by inductively coupled plasma-mass spectrometry on the ICP-QMS Agilent 7500CE spectrometer (Agilent Technologies, USA). Detailed description of the method was presented in previous studies22,27. Calibration solutions with a high range of elements’ concentration (from 0.1 μg/L to 100 μg/L) were used for the device calibration. The solutions were prepared with the international standard 2.74473.0100 “ICP Multi Element Standard Solution XXI CertiPUR” which contains the following elements: Ag, Al, As, Ba, Be, Bi, Ca, Cd, Co, Cr, Cs, Cu, Fe, Ga, In, K, Li, Mg, Mn, Na, Ni, Pb, Rb, Se, Sr, Tl, U, V, Zn, Hg. The concentration of all above-listed 24 elements in the milliQ, deuterium-depleted or heavy water did not exceed the upper detection limit (detection limit range – 0.1–10 ppm).
The ADSCs cultivation (in vitro live cell experiment)
The experiments with use of human cell culture in vitro were carried out in accordance with the human experiment issues of the Code of Ethics of the World Medical Association (Declaration of Helsinki). All procedures related to obtaining human biopsies, cell isolation and culturing were performed with written informed donor consent and in accordance with the laws of Ukraine. The study protocol was approved by the Bioethics Committee of the State Institute of Genetic and Regenerative Medicine NAMS of Ukraine (Kyiv, Ukraine). The cell culturing was carried out in the GMP/GTP-compliant biotechnological laboratory ilaya.regeneration (License to operate the Banks of human cord blood, other tissues and cells; issued by the Ministry of Health of Ukraine AE No. 186342 from 11.07.2018).
In all cases the voluntary informed consents were signed by donors of ADSCs. ADSCs samples (n = 6) were obtained from abdominal subcutaneous fat tissue of the healthy donors (3 female and 3 male) with normal somatometric and biochemical parameters without signs of obesity and viral or microbial infection. The age of patients was 23 ± 4.0 years. Body mass index (BMI) of adipose tissue donors was 20 ± 1.3.
The ADSCs were isolated from the lipoaspirate by enzymatic digestion in 0.1% collagenase IA and 0.1% pronase with 2% fetal bovine serum (FBS) (all - Sigma-Aldrich, USA) for 1 h at 37 °С. Detailed description of the method was presented in previous studies40,41,42,43,44,45,46. The obtained cell suspension was transferred to 25 cm2 cell culture flask (SPL, Korea) and cultured in the following growth medium: modified МЕМ-α (Sigma-Aldrich, USA) prepared from the powder diluted with milliQ water of natural isotope content supplemented with 10% FBS (Sigma-Aldrich, USA), 2mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin and 1 ng/ml bFGF-2 (all from Sigma-Aldrich, USA). The cells were cultured in multi-gas incubator CB210 (Binder, Germany) at +37 °C in the atmosphere of saturated humidity, 5% СО2 and 5% О2.
Before using in experement ADSCs were expanded until passage 3 and characterized according to criteria of International Society of Cellular Therapy42,43,44,45,47.
Flow cytometry for the ADSCs phenotype determination
The cell phenotype was assessed by flow cytometry (FACS) on BD FACSAria fluorescence-activated flow cell sorter-cytometer (BD Pharmingen, BD Horizon USA) and was performed in accordance to monoclonal antibodies manufacturer’s instructions (BD Pharmingen, BD Horizon USA). BD FACS Diva 6.1 software (BD Pharmingen, BD Horizon USA) was used for analysis. Detailed description of the method was presented in previous studies41,46.
Directed osteogenic differentiation of ADSCs
Osteogenic differentiation was performed in DMEM medium with low glucose (1 g/L) (BioWest, France) prepared from the powder by dissolution with milliQ water of natural isotope content and supplemented with addition of 10% FBS, 100 nM dexamethasone, 10 mM β-glycerophosphate, 500 μg/ml ascorbate-2-phosphate (all – Sigma-Aldrich, USA). After 21 days fixation and staining of cells with Alizarin Red S were carried out on mineral deposits. Detailed description of the method was presented in previous studies46.
Directed chondrogenic differentiation of ADSCs
The evaluation of chondrogenic differentiation of ADSCs was carried out using the micromass culture method. For this, 500 000 cells were centrifuged for 10 min at 400 g in 15 ml test tubes (Nunc, USA). Further, the cell precipitate was cultured in a chondrogenic induction medium containing DMEM with 4.5 g/L glucose (BioWest, France) prepared from the powder by dissolution with milliQ water of natural isotope content supplemented with addition of 50 μg/ml ascorbate-2-phosphate, 40 μg/L L-proline, 100 μg/ml pyruvate sodium, 10 ng/ml rhTGF-β3, 10–7 M dexamethasone (all – Sigma-Aldrich, USA), 1% ITS supplement (all – Gibco, USA), 1.25 mg/ml of bovine serum albumin, BSA (BioWest, France). Detailed description of the method was presented in previous studies46.
Directed adipogenic differentiation of ADSCs
The ADSCs were seeded with a density of 30 000 cells per 1 cm2. In 24 h the growth medium was changed on the serum-free adipogenic differentiation media with low, natural and high deuterium content: (1) phase I induction (4 days) – DMEM high glucose (4.5 g/L) (BioWest, France) prepared from the powder by dissolution with milliQ water of natural isotope content and supplemented with: 1 µM dexamethasone, 0.1 µM hydrocortisone, 50 µM indomethacin, 250 µM isobutylmethylxanthine, 0.2 nM triiodothyronine, 5 µg/ml insulin, 1% fatty-acid mixture, 0.01% bovine serum albumin (BSA), 1% ITS supplement, 50 µM ascorbate-2 phosphate, 2 mM L-glutamine, (all – Sigma-Aldrich, USA); phase II differentiation (10 days): media with same content without dexamethasone, indomethacin and isobutylmethylxanthine. Control adipogenic induction and differentiation media had a natural deuterium content. Experimental growth media had a composition similar to the control one, but was prepared on deuterated and deuterium-depleted waters. Gene expression, viability and metabolic activity of the cells were analyzed on days 3, 7 and 14 after induction to assess ADSCs adipogenic differentiation.
Cytochemistry, immunocytochemistry and histochemistry
Detailed description of the method was presented in previous studies46. Briefly, to confirm the osteogenic and adipogenic differentiation, the cells were fixed for 20 min in 10% buffered formalin (Sigma, USA), washed with DPBS (Sigma-Aldrich, USA) and stained for 20 min with 2% solution of Alizarin Red S (pH 4.1; for detecting calcified extracellular matrix deposits) or 0.5% solution of Oil Red O (for staining of neutral lipids) and Romanowsky-Giemsa stain for counterstaining, respectively (all – Sigma-Aldrich, USA).
The Oil Red O extraction was performed as described previously48. Measurements were performed on a LabSystems Multiskan PLUS spectrofluorometer (USA).
To confirm chondrogenic differentiation spheroids were removed from the incubator after 21 days of culturing, the medium was carefully aspirated, and spheroids were washed twice with DPBS. Spheroids were fixed in 10% buffered formalin for 2 hrs, dehydrated with ethanol, cleared with xylen and finally embedded in paraffin. Then, they were sectioned at 5 µm thickness. Slides were washed twice with distilled water and were stained by filtered Alcian Blue staining solution in the dark for 45 minutes at room temperature. Staining solution was removed and washed twice with PBS (all – Sigma-Aldrich, USA). Cartilage became stained an intense blue46.
The following primary antibodies were used for immunocytochemical staining: rabbit polyclonal against USP1 (Invitrogen, USA) and mouse monoclonal against PERILIPIN (R&D, USA). Secondary antibodies were donkey anti-mouse Alexa-488 and donkey anti-rabbit Alexa-647 conjugated (Thermo Fisher, USA). The cells were fixed for 20 minutes with cold 4% paraformaldehyde, permeabilized with intracellular staining for 15 min with 0.1% Triton X-100, blocked for 30 min in phosphate buffered saline with 0.1% Tween-20, 1% BSA, 5% FBS. The slides were incubated with primary antibodies overnight at 40 °C and with secondary antibodies for 1 hour at room temperature.
Cell viability
For the viability/cytotoxicity assessment, the cells were stained with РI (propidium iodide) (Sigma-Aldrich, USA) and FDA (fluorescein diacetate) (Sigma-Aldrich, USA) before differentiation and after 3, 7 and 14 days of adipogenic differentiation. Detailed description of the method was presented in previous studies40,41. The number of dead and living ADSCs in different groups was counted using inverted fluorescent microscopy AxioObserver A1 and ZEN 2012 software (Carl Zeiss). The cell viability was calculated as the ratio of living cells to the total number of cells and was expressed as a percentage according to the formula:
The ADSCs metabolic activity
Detailed description of the method was presented in previous studies41,49. ADSCs metabolic activity was assessed on days 3, 7 and 14 of adipogenic differentiation. 10% of Alamar Blue (redox indicator; Thermo Fisher, USA) was added to the culture medium and incubated for 3 h according to manufacturer’s instructions. Reduced Alamar Blue was detected at 540 nm vs 630 nm at LabSystems Multiskan PLUS spectrofluorometer (USA).
The RT-qPCR assay
Detailed description of the method was presented in previous studies46. Total RNA was isolated from ADSCs using NucleoZOL (MACHEREY-NAGEL GmbH & Co. KG, Germany) according to manufacturer’s protocol. The RNA quality and concentration were determined with a spectrophotometer NanoDrop 1000 (Thermo Scientific, USA). 2 μg of isolated RNA were reverse transcribed to cDNA using RevertAid First Strand cDNA Synthesis kit (Thermo Scientific, USA). RT-qPCR was performed with a 7500 Real-Time PCR System (Applied Biosystems, CA, USA) using 5× HOT FIREPol EvaGreen qPCR Mix Plus (ROX) (Solis BioDyne, Estonia). The Applied Biosystems 7500 system software (V. 1.3.1) was used for data analysis. The primers sequences are listed in Table 1. The following PCR cycling conditions were applied: 10 min at 95 °C, 40 cycles of 10 s at 95 °C, and 40 min at 60 °C. Expression level of TATA-box binding protein (TBP) was used as internal control. Ct values for target genes were normalized against Ct value of TBP at the same threshold level. The relative quantification (comparative Ct (ΔΔCt) method) was used to compare the expression levels of the target genes with the internal control. Dissociation curve analysis was performed after every run to check the primers specificity. Results were presented in relative units. For all conditions, reaction was performed three times (each gene in triplicate). GraphPad Prism 4 (GraphPad Software, USA) and MS Excel were used for statistical analysis and graphic data presentation.
Adipokine production
After differentiation ADSCs in adipocytes, the content of the following 10 adipokines, cytokines and chemokines were determined in the incubation medium by the method of flow fluorimetry (multiplex analysis, Luminex xMAP): LEPTIN, ADIPONECTIN, ADIPSIN, VASPIN, CHEMERIN, TNF-α, IL-6, IL-8, IL-10, МСР-1, IP-10. This method included a multiplex immune reaction that took place on the various-diameter microparticles carrying the absorbed antibodies, and subsequent flow fluorescence analysis as well as simultaneous measurement of cytokine concentration. The procedures were conducted according to the Bio-Plex Pro Assay protocol. The results were recorded using an automatic photometer for Bio-Plex microplates (Bio-Plex 200 Systems, Bio-Rad, USA) and the Bio-Plex Manager (“Bio-Rad”) software. The test substances concentration was determined from the calibration curve for each cytokine (the dynamic range 2–32 000 pg/mL) according to the manufacturer recommendations. Detailed description of the methods was presented in previous studies46.
Microscopy
Microscopy examinations of live cells, cell cultures and cytological slides were carried out with inverted AxioObserver A1 microscope equipped with an AxioCam ERc 5 s digital camera (all – Carl Zeiss, Germany) and ZEN 2012 software.
Statistics
The data were reported as mean ± SD for each group. Statistical analyses were performed using one-way analysis of variance (ANOVA) in Origin Pro software. Differences were considered to be statistically significant when p < 0.05.
Results
Preparation and characterization of ADSCs
Subcutaneous fat is a convenient source for ADSCs obtaining50. The possibility of in vivo converting white adipocytes of subcutaneous adipose tissue into beige/brown adipocytes was previously shown51. Thus, the in vitro study of ADSCs differentiation from subcutaneous adipose tissue is a valuable tool in the search for new substances targeted on adipocyte function and in the development of new methods for obesity, metabolic syndrome and T2DM treatment.
Cells obtained from human lipoaspirate adherent were expanded in vitro until P3 and before experiments were characterized according to the ISCT position papers for compliance to minimal defined criteria of MSCs and ADSCs47.
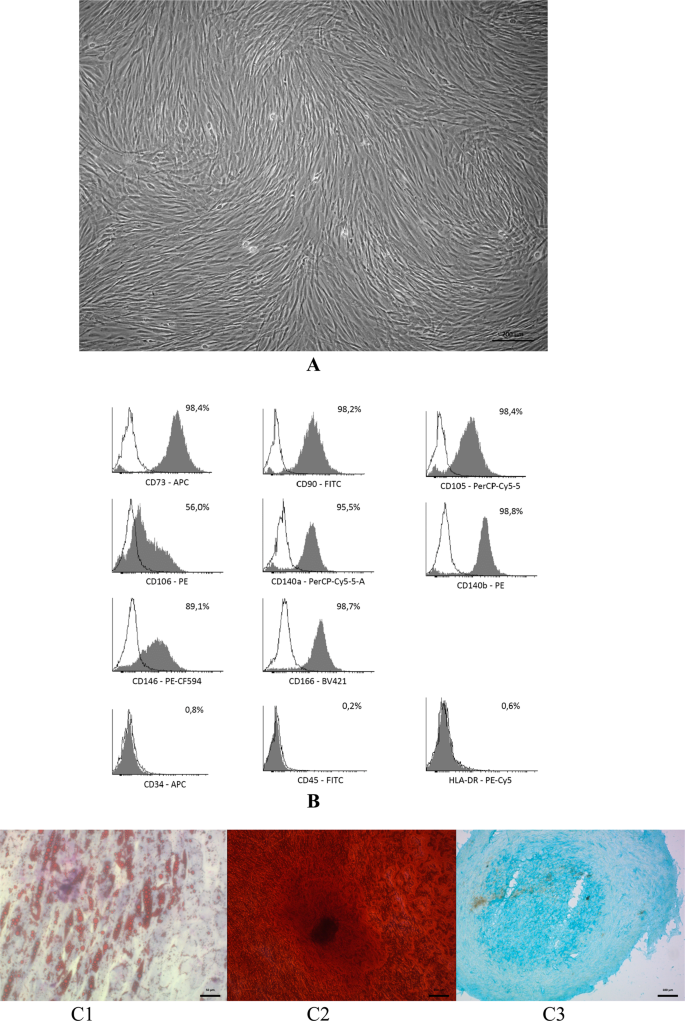
After expansion over three passages, the cells obtained from lipoaspirate presented homogeneous population of cells with fibroblast-like morphology (Fig. 1A). The study of ADSCs immunophenotype showed that they expressed characteristic positive stem cell markers and did not express negative markers (Fig. 1B).
Сharacterization of ADSCs. (A) The ADSCs morphology at P3 before directed multilineage differentiation. Phase-contrast microscopy. Bar scale – 200 µm. (B) Representative FACS histograms the ADSCs immunophenotype at P3. (C) The ability to direct three-lineage differentiation of ADSCs in vitro. C1 – adipogenic differentiation, Oil Red and Romanovsky-Giemsa stain, the bar = 50 µm; C2 – osteogenic differentiation, Alizarin Red S stain, the bar = 100 µm; C3 – chondrogenic differentiation, Alcian Blue stain, bar scale = 100 µm.
Also, ADSCs demonstrated key characteristic features of MSCs – the ability to directed orthodoxic three-lineage differentiation in vitro. So, after 14 days of cultivation in adipogenic differentiation medium, the fibroblast-like cells were converted into adipocytes containing lipid vacuoles (Fig. 1C1). The ADSCs also demonstrated ability to osteogenic differentiation and production of mineralized extracellular matrix. Alizarin Red S staining showed a positive reaction to the Ca content at day 21 after differentiation (Fig. 1C2). As for the chondrogenic differentiation, a dense chondroid was obtained after 21 days of chondrogenic induction (Fig. 1C3).
Thus, ADSCs isolated from lipoaspirate, complied the minimal ISCT criteria for MSCs, such as adherence to plastic in standard culture conditions, fibroblast-like morphology, typical phenotype and ability for directed orthodoxic multilineage differentiation in vitro.
To find out whether deuterium could be involved in regulation of adipogenesis, we composed adipogenic differentiation media on the base of deuterium-depleted and deuterated water. The adipogenic differentiation medium, that was made with milliQ water, served as control.
Directed adipogenic differentiation of ADSCs with various deuterium content
Differentiation of ADSCs into adipocytes is a complex multi-step process in which the stages of induction/commitment (adipocyte progenitors, days 0–3), early differentiation (pre-adipocytes, days 3–7) and terminal differentiation (mature adipocytes, days 7–14) can be distinguished.
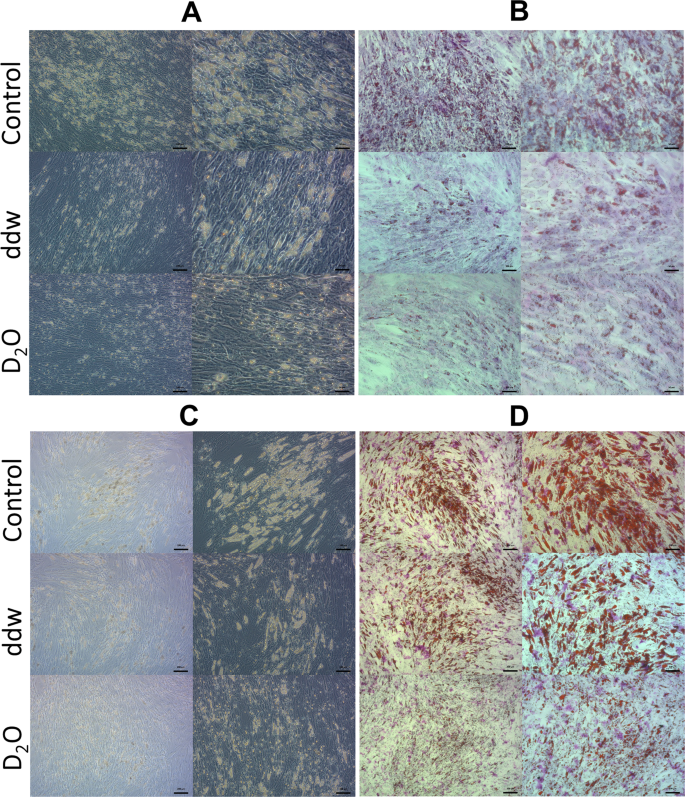
After 7 days of cultivation in the adipogenic differentiation medium, cells began to accumulate lipid vacuoles in all groups (Fig. 2A,B). However, there were noticeable differences in number, size and form of the lipid vacuoles in ADSCs that underwent differentiation in deuterated and deuterium-depleted inductive media compare to control. A significant number of cells with lipid vacuoles were observed in the control group, and only single adipocytes in the experimental groups. At the same time, the process of differentiation had no morphological differences between groups and followed the path of adipocyte formation with the fusion of the initial small lipid vacuoles into one or two large vacuoles located centrally in the cytoplasm, which is typical for white adipocytes.
Adipogenic differentiation of ADSCs in media with various deuterium concentration. (A) Phase-contrast microscopy 7 days, (B) Light microscopy Oil Red O dye 7 days, (C) Phase-contrast microscopy 14 days, (D) light microscopy Oil Red O dye 14 days). Control – differentiation medium was made on the base of milliQ water; ddw – differentiation medium was made on the base of deuterium-depleted water; D2O – differentiation medium was made on the base of deuterated water. In each section: left column – the bar = 100 µm; right column – the bar = 50 µm.
But on the 14th day there were no such a striking difference except for the group of deuterated differentiation medium, where lipid vacuoles were much smaller compared to other groups (Fig. 2C,D). As for the cells in deuterium depleted medium, they almost reached the control ones.
At the same time, on the 14th day, significant morphological differences were observed between differentiated from ADSCs adipocytes in all groups. Thus, in the control group (natural deuterium content) and in the group with high deuterium content, predominantly unilocular adipocytes containing one or two large lipid vacuoles were formed. As indicated earlier, this morphological trait is a characteristic feature of white adipocytes. In the group with low deuterium content, single unilocular adipocytes were also found. However, most adipocytes had numerous small vacuoles located on the periphery of the cytoplasm. This morphology is characteristic for brown and beige adipocytes.
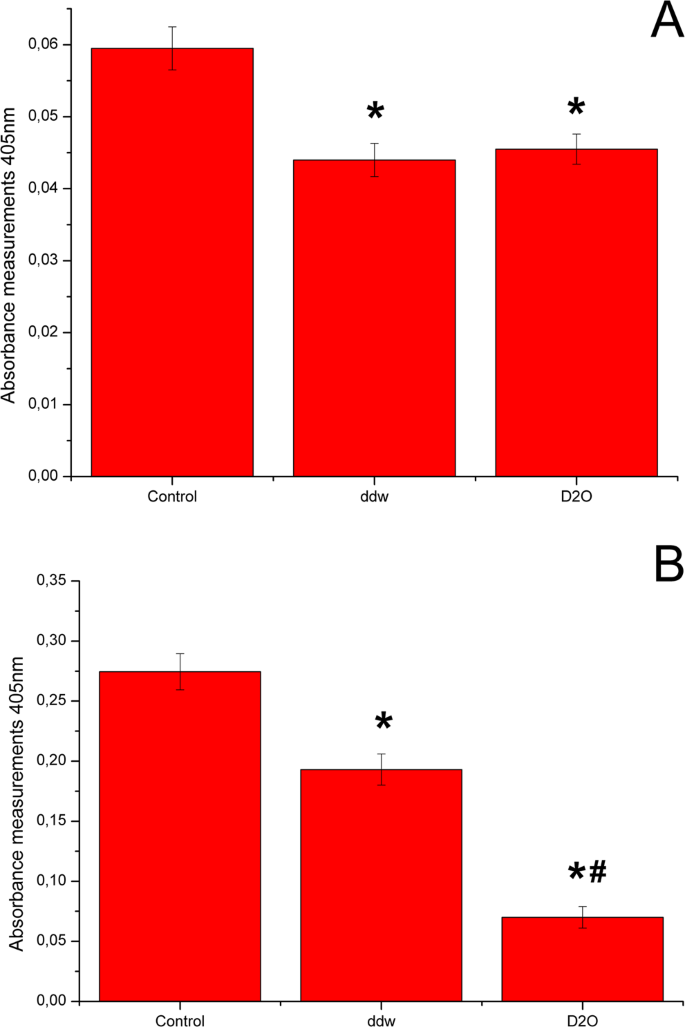
The adipogenic differentiation efficiency was evaluated by Red Oil O dye extraction on the 7th аnd 14th days of the experiment (Fig. 3). Both high and low doses of deuterium inhibited adipogenic differentiation of ADSCs. On day 7, inhibition of adipogenic differentiation of ADSCs was the same in deuterium-depleted and deuterated media (Fig. 3A). However, adipogenic media on the base of deuterated water showed higher differentiation inhibition on day 14 (Fig. 3B).
Diagrams represent results of Oil Red extraction after 7 (A) and 14 (B) days cultivation in adipo-inductive media with various deuterium content. Control – differentiation medium was made on the base of milliQ water; ddw – differentiation medium was made on the base of deuterium-depleted water; D2O – differentiation medium was made on the base of deuterated water. (The results are expressed as mean ± SD (n = 6), significant differences between groups: *p < 0.05 compared to control group, #p < 0.05 compared to ddw group).
ADSCs gene expression profile in process of adipogenic differentiation in media with various deuterium content
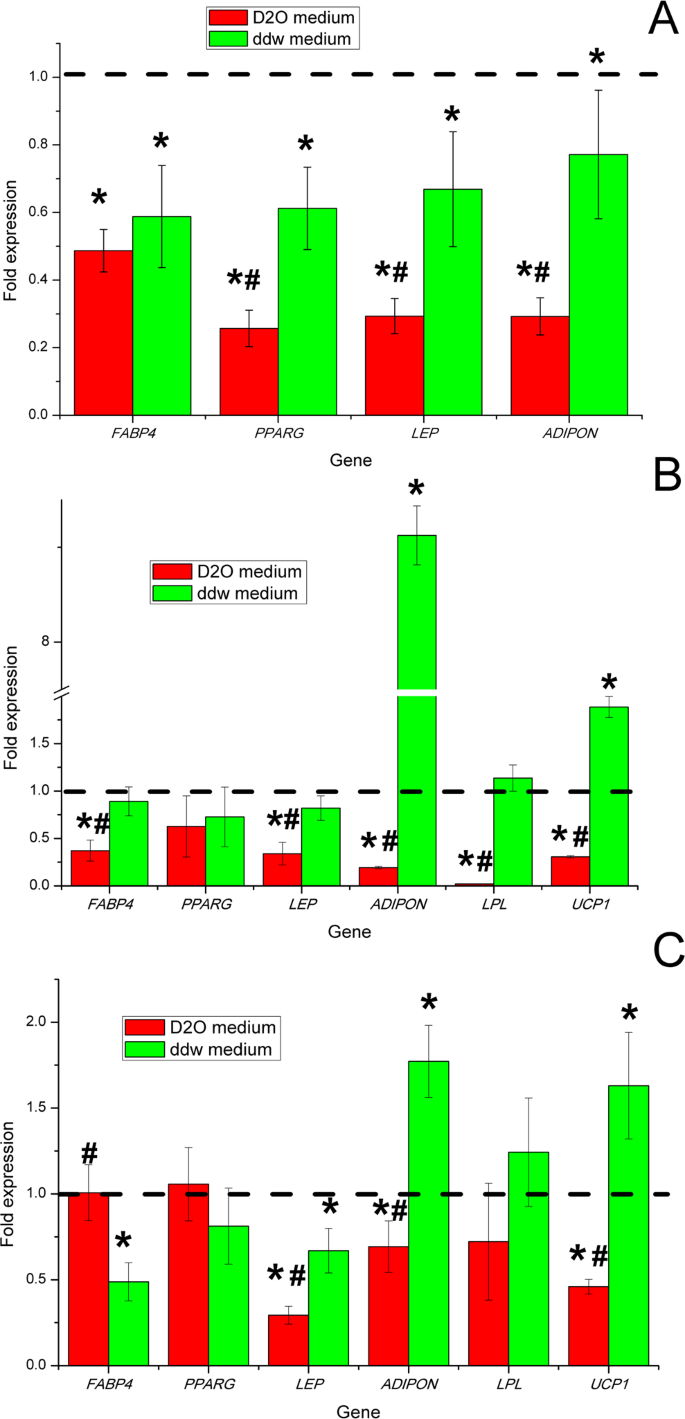
To obtain further insight into the molecular mechanisms of ADSCs during adipogenic differentiation in differentiation media with various deuterium content, we evaluated the mRNA expression levels of the key genes linked with adipogenesis. Evaluation of gene expression by cells was performed before the experiment and on days 3, 7 and 14. (Fig. 4). mRNA expression of all genes was normalized to TBP expression level. The fold-change was calculated over the control conditions that correspond to 1.
Diagrams represent results of the mRNA expression level of adipogenic markers in ADSCs after adipogenic differentiation in media with various deuterium content after 3 (A), 7 (B) and 14 (C) days. Control – differentiation medium was made on the base of milliQ water, marked with a dotted line; ddw – differentiation medium was made on the base of deuterium-depleted water; D2O – differentiation medium was made on the base of deuterated water. (The results are presented as mean ± SD (n = 6), significant differences between groups: *p < 0.05 compared to control group, #p < 0.05 compared to ddw group).
The ADSCs did not express the studied key adipogenesis genes in culture in vitro before differentiation (data not showed). At day 3 after starting differentiation ADSCs began to express master-gene of adipogenesis PPARG and other characteristics for adipocytes genes: FABP4 (encodes carrier protein for fatty-acid), LEP and ADIPON (key adipokines that in vivo regulate satiety, hunger and various aspects of energy metabolism in human body). At this point, expression of LPL and UCP1 genes was not detected, which coincided with morphological signs of the formation of immature white adipocytes. Thus, at the induction/commitment stage, the expression of adipogenesis genes was statistically significantly higher in the control group with natural deuterium content (Fig. 4A). According to the data on gene expression in groups with low and high deuterium content, inhibition of the induction/commit process of ADSCs in the adipogenic direction occurred.
Based on gene expression analysis, at day 7th ADSCs adipogenic differentiation in deuterium-depleted medium was only partially inhibited comparing to control differentiation medium. Namely, the mRNA expression level of the key master regulator of adipogenic differentiation nuclear receptor PPARG and marker of pre-adipocytes and adipocytes FABP4 did not change in ADSCs differentiated under the influence of deuterium depleted water (Fig. 4B). Moreover, a statistically significant up-regulation of expression UCP1 and ADIPON genes occurred in the group with low deuterium content compared with the control group and the group with high deuterium content (Fig. 4B). In the group with high deuterium content, significantly lower level of expression of all the studied genes was observed, which indicated inhibition of adipogenic differentiation.
On day 14th, the level of PPARG and FABP4 genes expression in the group with high deuterium content reached a level comparable to the groups with natural and low deuterium content. Moreover, the level of gene expression of the key adipokines LEP and ADIPON was statistically significantly lower than in groups with natural and low deuterium content. Statistically significant increase in the level of UCP1, LPL and ADIPON genes expression and lower level of FABP4 and LEP genes expression compared to the control group on the 14th day was observed. In total, this may reflect the switching the program of adipogenesis in the group with a low deuterium content to the formation of functional thermogenic brown/beige adipocytes.
Detection of UCP1 expression on protein level by immunocytochemistry
In order to confirm the expression of marker proteins of brown/beige adipocytes at the protein level we performed an immunocytochemical study (Fig. 5).
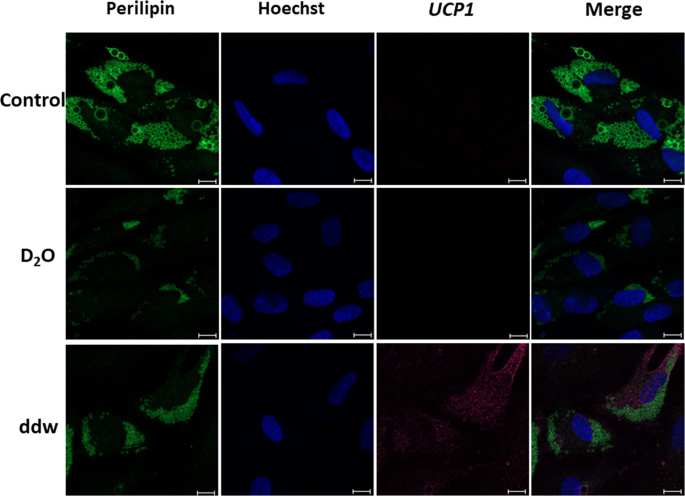
Immunocytochemical detection of UCP1 in adipocytes, differentiated from ADSCs in media with different deuterium content. Control – differentiation medium was made on the base of milliQ water; ddw – differentiation medium was made on the base of deuterium-depleted water; D2O – differentiation medium was made on the base of deuterated water. In each section – the bar = 50 µm. Fluorescent microscopy.
According to the results of immunocytochemical analysis on the 14th day of ADSCs differentiation using our serum-free adipogenic medium, adipocytes formed and that was confirmed by detection of the adipocyte-specific protein PERELIPIN (forms the membrane of lipid vacuoles). Moreover, only in group with a low deuterium content UCP1 (a marker of thermogenic brown/beige adipocytes) was detected at the protein level. So, in the group with the natural deuterium content in our serum-free differentiation protocol, the formation of white adipocytes occurred, what was confirmed by the morphological features described earlier (the formation of adipocytes with one or two large lipid vacuoles). In the group with high deuterium content white adipocytes also developed. However, it was noted significant inhibition and delay in time of the adipogenic differentiation process.
The adipokine production by adipocytes differentiated from ADSCs in media with different deuterium content
Multiplex analysis of 11 key produced adipokines showed that, despite the similar general qualitative spectrum of products, there are statistically significant differences in the quantitative production of cytokines (Table 2). It is important to note that in the group with low deuterium content we observed an increased production of obesity-protective adipokines (LEPTIN and ADIPONECTIN) and IL-6 (this cytokine increases the production of LEPTIN in adipocytes), and decreased production of pro-inflammatory TNF-α, IL-8, MCP-1, IP-10, ADIPSIN (inhibits the lipolysis) and IL-10 (inhibits the thermogenesis and browning). Despite the fact that adipocytes with morphological and phenotypic signs of white adipocytes developed in groups with natural and high deuterium content, statistically significant quantitative differences in the production of all adipokines were also observed between them. It can be explained by significant inhibition and lag of the adipogenesis process in an environment with increased deuterium content.
Viability and metabolic activity of ADSCs in the process of adipogenesis in differentiation media with various deuterium content
To clarify the question whether deuterium-mediated inhibition of ADSCs adipogenic differentiation was associated with cytotoxic effect of deuterium we analyzed viability and metabolic activity of differentiated ADSCs.
Viability of ADSCs in inductive media with various deuterium content
The cell viability/cytotoxicity was assessed with FDA and PI staining ADSCs before differentiation and during differentiation in adipogenic media with various deuterium content on 3, 7 and 14 days of cultivation (Table 3). The cell viability in culture of ADSCs in growth medium before differentiation was 97.3 ± 3.4% according to FDA/PI staining. On the 3rd day of differentiation, a statistically significant decrease in viability was observed in all groups, which can be caused by apoptosis and necrosis of part of the cells upon transition to both serum-free culturing conditions and apoptosis under the influence of differentiation factors. It is important to note that in the group with a high deuterium content on the 3rd day of the adipogenesis, there was a sharp decrease in cell viability, which was probably due to the cytotoxic effect of deuterium in the differentiating microenvironment. On the 7th and 14th days in a group with a high deuterium content, gradual increase in cell viability occurred, probably due to the adaptation of cells to a high deuterium content in the microenvironment.
Metabolic activity of ADSCs in inductive media with various deuterium content
Alamar Blue assay was performed on 3rd, 7th and 14th day of ADSCs differentiation in adipogenic media with various deuterium content (Fig. 6). The metabolic activity of undifferentiated ADSCs cultured in growth medium was set at 100%, and their changes are given as percentage of controls.
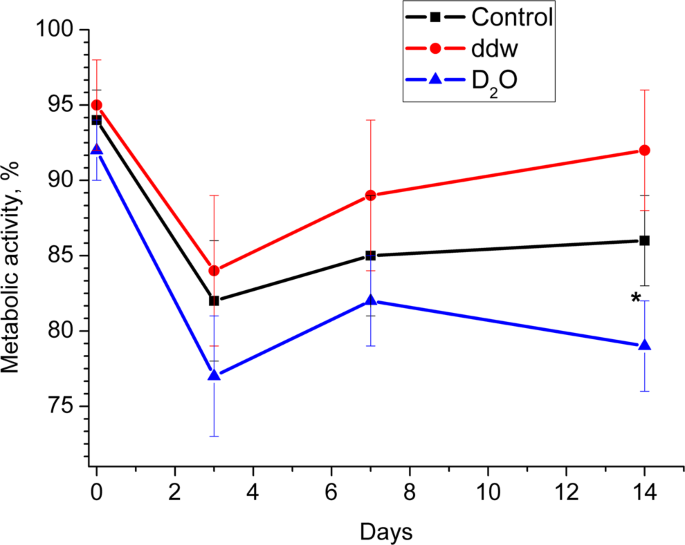
Diagrams represent results of the Alamar Blue assay (Metabolic activity) in media with various deuterium content after 0, 3, 7 and 14 days. Control – differentiation medium was made on the base of milliQ water; ddw – differentiation medium was made on the base of deuterium-depleted water; D2O – differentiation medium was made on the base of deuterated water. (The results are expressed as mean ± SD (n = 6), significant differences between groups: *p < 0.05 compared to control group).
A decrease in metabolic activity in all groups on the 3rd day of adipogenesis was observed, what may be both due to switching the cells from growth (proliferation) processes to differentiation, and to adaptation to serum-free conditions. Subsequently, in groups with a natural and low deuterium content, an increase in metabolic activity occurred, especially pronounced in the group with low deuterium content. This fact may reflect the differentiation of ADSCs in brown/beige adipocytes when using our cocktail of differentiating factors in a microenvironment with low deuterium content. Importantly, metabolic activity was reduced in the group with a high deuterium content compared to the group with a natural and low deuterium content during the entire adipogenesis. It correlates with morphological characteristics and gene expression data and probably can be explained by the cytotoxic effect of deuterium at the induction stage/committing cells and its inhibitory effect on the differentiation of ADSCs.
Discussion
Obesity, metabolic syndrome and T2DM became a global public health problem in the 21st century. One of the key links in the pathogenesis of the development of these diseases is adipose tissue. In the last 10 years, the phenomenon of the existence of active thermogenic beige adipose tissue in adult humans was discovered. The possibility of in vivo conversion of subcutaneous white adipocytes into beige adipocytes was further shown. These facts, in total, in addition to upcoming pharmacological strategies for inhibiting the development of adipose tissue, can also be considered as a promising approach for the prevention and treatment of obesity, metabolic syndrome and T2DM by the formation of beige adipose tissue de novo and/or by the conversion of existing subcutaneous white adipocytes into beige adipocytes.
In our study, the directed adipogenic differentiation of ADSCs with a two-step (induction + maturation) non-serotonous protocol based on the use of various hormones and small molecules led to the formation of classical white adipocytes in a microenvironment with a natural and high deuterium content. Interestingly, adipogenesis in a low deuterium content is diverted towards the development of brown/beige adipocytes under the influence of the same induction and differentiation factors. The differentiation of ADSCs in brown/beige adipocytes in microenvironment with low deuterium content was confirmed by morphological features, gene expression, UCP1 protein detection. Moreover, despite the fact that the resulting brown/beige adipocytes produced the same spectrum of adipokines as their counterparts (white adipocyte) obtained in the process of differentiation of ADSCs in microenvironment with natural and high deuterium content, but there were significant quantitative differences. In general, brown/beige adipocytes showed increased production of anti-obesity and lower production of pro-inflammatory adipokines. Thus, in addition to forming of beige adipocytes, which are functionally useful for the prevention and treatment of obesity, metabolic syndrome and T2DM, we also discovered the secretion of a protective spectrum of adipokines, which can provide additional benefits at the systemic level of the whole body (organism).
Despite the difference of morphology and resulting type of differentiated from ADSCs adipocytes (brown/beige vs white), both ddw and D2O groups inhibited adipogenic differentiation as confirmed by gene expression and Red Oil O extraction assay. However, the inhibition of adipogenesis in D2O group was drastic which can be partially explained by acute deuterium cytotoxicity. In our previous study on the effect of deuterated and deuterium-depleted water on the proliferation and migration of ADSCs, the acute cytotoxic effect of high deuterium concentrations was also noted41. An interesting question for further research is to establish the mechanism of cell death (necrosis vs apoptosis) with high deuterium content, as well as the role of mitochondria in cell death. A comparative study of the mechanisms of cell death in conditions that promote growth (proliferation) and differentiation is also interesting. In case of ddw group, cell viability was not affected. This is in line with our previous observations as well as with other authors’ reports41,52,53,54,55.
At the same time, deuterium excess or depletion had opposite effect on the cellular metabolism: differentiated ADSCs metabolic activity was increased in ddw group and inhibited in D2O group (Fig. 6). This also corresponds to our earlier data on the effect of low and high deuterium content on the metabolic activity of ADSCs in growth conditions41.
High concentrations of deuterium in the environment can lead to inhibition of many reactions associated with the energy component in the chain of biochemical reactions20,22,23. This can be explained by the fact that hydrogen is the main participant in electron transfer and is a reducing agent in many biochemical cascades30,35,37. Therefore, stronger deuterium bonds, when replacing protium, will lead to inhibition of such reactions.
As for deuterium depletion, after classical ideas about the dilution of substances56, changes in the metabolic rate should be negligible at deuterium concentration below 150 ppm. However, the observed effects are significant57. This suggests that deuterium acts as an element, which is necessary as a rate regulator of biochemical reactions cascades. On the other hand, deuterium could be considered as an element that affects the substance chirality. That explains the mechanism of changes in many system parameters as a result of different D/H ratios22,27,41. In other words, the presence of deuterium or protium in a substance leads to various ways of further reactions. Accordingly, the entire metabolism can go in different (and unpredictable yet) directions (at different rates) depending on the presence of deuterium or protium in the initial or intermediate substance.
In order to find out some mechanisms of the action of deuterium on the ADSCs adipogenic differentiation, we examined the mRNA expression of some adipogenesis markers.
So, peroxisome proliferator-activated receptor gamma (PPAR-γ or PPARG) is a master gene (master regulator) of adipogenic differentiation and encodes a type II nuclear receptor. PPARG is mainly presented in adipose tissue, colon and macrophages and regulates fatty acid storage and glucose metabolism58,59,60. Here we did not observe significant changes in the PPARG expression in all comparison groups. But we observed inhibition of express PPARG gene at the stage of induction of adipogenesis in groups with both high and low deuterium content. Moreover, at the end of the differentiation process, the level of expression increase, which may be explained by the asynchrony of the differentiation process in the ADSCs population at the single cells level.
FABP4 (fatty acid binding protein 4) or aP2 (adipocyte Protein 2) is a carrier protein for fatty acids that is primarily expressed in adipocytes and macrophages61. Blocking this protein is perspective for treating various diseases62,63,64,65,66. After our data, FABP4 expression was slightly reduced in both groups at the same time the level of PPARG gene expression reflected the overall delay in the process of ADSCs differentiation.
Leptin (LEP) is a hormone predominantly made by adipose cells that helps to regulate energy balance by inhibiting hunger. Leptin expression level in both groups was slightly lower than in the control group. This suggests that the level of cell metabolism, namely, the absorption of lipids, takes place at low intensity. A high concentration of LEP in the medium with reduced expression of the leptin gene compared with the control group can be explained by different rates of leptin secretion, which, as is known from work67, is independent of the regulation of leptin mRNA expression due to the presence of vesicular leptin depots in adipocytes68. Moreover, since adipocytes themselves express leptin receptors69, hypothetically, this difference can also be associated with differences in autocrine signaling between different adipocyte subtypes.
Adiponectin (ADIPON) is a hormone that regulates glucose metabolism and fatty acid oxidation70. Some studies have shown that ADIPON was inversely correlated with body weight index71. Studies of mice with elevated levels of adiponectin showed a decrease in adipocyte differentiation and increase in energy costs, which was due to the uncoupling of mitochondria72. It has also been shown that adiponectin and leptin alter insulin sensitivity in mice73. High ADIPON expression in ddw group could be associated with high metabolism level and high mitochondrial activity in context of ADSCs differentiation in this condition into beige adipocytes35,37,41. Another cause of high adiponectin expression could be a compensatory effect to low leptin expression as these two proteins act synergistically73,74. The low ADIPON expression in the D2O group may be associated with both general inhibition of the adipogenesis process and deviation of the direction of differentiation into white adipocytes with switching to energy storage by phosphorylation of fatty acids75.
Lipoprotein lipase (LPL) is a water-soluble enzyme that hydrolyzes triglycerides in lipoproteins. It is also involved in promoting the cellular uptake of chylomicron remnants, cholesterol-rich lipoproteins, and free fatty acids76,77,78. In the deuterated medium, we did not observe LPL expression on day 7th of the experiment, and, it was significantly lower on day 14th. This may be explained by deuterium-triggered metabolic changes which led to the formation of white adipocytes. In the ddw medium there was no difference with the control group.
UCP1 (uncoupling protein 1, also known as thermogenin)79,80,81 is an uncoupling protein found in the mitochondria of beige adipose tissue (BAT). Heat production using UCP1 in beige adipose tissue occurs with the separation of cellular respiration and phosphorylation, that is the rapid oxidation of nutrients occurs with a low intensity of ATP production82. According to our data, the decreased expression of UCP1 in a deuterated medium confirms that differentiation of ADSCs in this condition goes towards white adipocytes. High expression of UCP1 in ddw medium seems to be the result of the ADSCs differentiation into functional beige adipocytes. Consequently, the main mechanism of the inhibitory/stimulating effect of high/low deuterium concentrations should be sought in the work of the respiratory chain and the mitochondrial complex itself.
Taking into account the fact that ddw directs the differentiation of adipocytes into their other subtype (brown/beige vs white adipocytes), the apparent “inhibition” of adipogenesis in the ddw group compared to the control medium actually reflects the differences in the formation of different adipocyte subtypes. Whereas in the case of a group with a high deuterium content compared with the control, the inhibition process of differentiation of white adipocytes from ADSCs actually occurs.
In our study, we first obtained data on the effect of various deuterium concentrations on the efficiency and direction (brown/beige vs white adipocytes) of adipogenic ADSCs differentiation in an in vitro model system. For the possible practical application of these results, additional studies are needed that would allow a more detailed description of the molecular mechanisms of influence of deuterium various concentrations at the cellular level, as well as studies at the body level. In further experiments on laboratory animals, it is necessary to study the effect of low concentrations of deuterium on the functioning of various fat depots in various physiological and pathological situations (stress, high-fat diet, etc.)83,84.
Altogether, our data revealed the importance of D/H ratio in culture medium for directed adipogenic differentiation of human ADSCs. However, the mechanisms that explain involvement different deuterium concentration in regulation ADSCs adipogenic differentiation are not clarified and this opens a field for the future research.
Conclusion
We have demonstrated that both deuterium depleted and deuterated inductive media inhibit adipogenic differentiation of human ADSCs compared to medium with normal deuterium content. The inhibitory effect of deuterated medium could be partially explained by its increased cytotoxicity. However, surprisingly, with the same instructional signals (hormones and small molecules), the differentiation of ADSCs in the microenvironment with a low deuterium content deviated in direction of brown/beige adipocytes development. Thus, the separate or combined use of ddw and D2O may be used in the complex treatment of obesity, metabolic syndrome and T2DM.
Data availability
The data used to support the findings of this study is available from the corresponding author upon request.
References
-
Qatanani, M. & Lazar, M. A. Mechanisms of obesity-associated insulin resistance: many choices on the menu. Genes Dev. 21, 1443–1455 (2007).
-
Golay, A. & Ybarra, J. Link between obesity and type 2 diabetes. Best Pract. Res. Clin. Endocrinol Metab. 19, 649–663 (2005).
-
Novelli, E. L. B. et al. The adverse effects of a high-energy dense diet on cardiac tissue. J. Nutrition Environ. Med. 12, 287–290 (2002).
-
Bianchini, F., Kaaks, R. & Vainiuo, H. Overweight, obesity and cancer risk. Lancet Oncology 3, 565–574 (2002).
-
World Health Organization, WHO, Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation (WHO Technical Report Series 894), http://www.who.int/nutrition/publications/obesity/WHO_TRS_894/en/ (2016).
-
Dinsa, G. D., Goryakin, Y., Fumagalli, E. & Suhrcke, M. Obesity and socioeconomic status in developing countries: a systematic review. Obes Rev. 13(11), 1067–1079, https://doi.org/10.1111/j.1467-789X.2012.01017.x (2012).
-
Peiris, A. N., Struve, M. F., Mueller, R. A., Lee, M. B. & Kissebah, A. H. Glucose metabolism in obesity: influence of body fat distribution. J. Clin. Endocrinol. Metab. 67(4), 760–767 (1988).
-
Halenova, T. et al. P62 plasmid can alleviate diet-induced obesity and metabolic dysfunctions. Oncotarget 8(34), 56030–56040 (2017).
-
Halenova, T. et al. Effect of C60 fullerene nanoparticles on the diet-induced obesity in rats. Int. J. Obesity. 42, 1987–1998 (2018).
-
Green, C. J. & Hodson, L. The influence of dietary fat on liver fat accumulation. Nutrients. 6(11), 5018–5033 (2014).
-
Farrigan, C. & Pang, K. Obesity market overview. Nat. Rev. Drug Discov. 1(4), 257–258, https://doi.org/10.1038/nrd781 (2002).
-
Catrysse, L. & van Loo, G. Adipose tissue macrophages and their polarization in health and obesity. Cell Immunol. 330, 114–119, https://doi.org/10.1016/j.cellimm.2018.03.001 (2018).
-
Kleinendorst, L., van Haelst, M. M. & van den Akker, E. L. T. Genetics of Obesity. Exp. Suppl. 111, 419–441, https://doi.org/10.1007/978-3-030-25905-1_19 (2019).
-
Lizcano, F. The beige adipocyte as a therapy for metabolic diseases. Int. J. Mol. Sci. 20(20), pii:E5058; https://doi.org/10.3390/ijms20205058 (2019).
-
Brown, A. C. Brown adipocytes from induced pluripotent stem cells-how far have we come? (Ann NY Acad. Sci.) https://doi.org/10.1111/nyas.14257 (2019).
-
Villarroya, J. et al. New insights into the secretory functions of brown adipose tissue. J Endocrinol. pii: JOE-19-0295.R1; https://doi.org/10.1530/JOE-19-0295 (2019).
-
Lee, J. H. et al. The role of adipose tissue mitochondria: regulation of mitochondrial function for the treatment of metabolic diseases. Int. J. Mol. Sci. 20(19), pii:E4924; https://doi.org/10.3390/ijms20194924 (2019).
-
Nissensohn, M., Castro-Quezada, I. & Serra-Majem, L. Beverage and water intake of healthy adults in some European countries. Int. J. Food Sci. Nutr. 64(7), 801–805, https://doi.org/10.3109/09637486.2013.801406 (2013).
-
Bylund, J. et al. Measuring sporadic gastrointestinal illness associated with drinking water - an overview of methodologies. J. Water Health. 15(3), 321–340, https://doi.org/10.2166/wh.2017.261 (2017).
-
Atzrodt, J., Derdau, V., William, J. & Reid, M. Deuterium- and Tritium-Labelled Compounds: Applications in the Life Sciences. Angew. Chem. Int. Ed. 57, 1758–1784 (2018).
-
Somlyai, G. Defeating Cancer! The Biological Effects of Deuterium Depletion. (Bloomington: Author House, 2002).
-
Syroeshkin, A. V. et al. D/H control of chemical kinetics in water solutions under low deuterium concentrations. Chem. Eng. J. 377, 119827, https://doi.org/10.1016/j.cej.2018.08.213 (2019).
-
Robins, R. J., Remaud, G. S. & Billault, I. Natural mechanisms by which deuterium depletion occurs in specific positions in metabolites. Eur. Chem. Bull. 1(1), 39–40 (2012).
-
Zhang, K. et al. Lack of deuterium isotope effects in the antidepressant effects of (R)-ketamine in a chronic social defeat stress model. Psychopharmacology. 235, 3177–3185, https://doi.org/10.1007/s00213-018-5017-2 (2018).
-
Charidemou, E., Ashmore, T. & Griffin, J. L. The use of stable isotopes in the study of human pathophysiology. Int. J. Biochem. Cell Biol. 93, 102–109 (2017).
-
Mosin, O. & Ignatov, I. Biological Influence of Deuterium on Prokaryotic and Eukaryotic cells. J. Med. Physiol. Bioph. 1, 52–72 (2014).
-
Syroeshkin, A. V. et al. The effect of the deuterium depleted water on the biological activity of the eukaryotic cells. J. Trace Elem. Med. Biol. 50, 629–633 (2018).
-
Strekalova, T. et al. Deuterium content of water increases depression susceptibility: The potential role of a serotonin-related mechanism. Behav. Brain Res. 277, 237–244 (2015).
-
Dzhimak, S. S., Basov, A. A. & Baryshev, M. G. Content of Deuterium in Biological fluids and organs: influence of deuterium depleted water on D/H gradient and the process of adaptation biochemistry. Bioph. Mol. Biol. 465, 370–373 (2015).
-
Pomytkin, I. A. & Kolesova, O. E. Relationship between Natural concentration of heavy water isotopologes and rate of H2O2 generation by mitochondria. Bul. Exp. Biol. Med. 142(5), 570–572 (2006).
-
Krempels, K., Somlyai, I., Somlyai, G., Somlyai, I. & Somlyai, G. A Retrospective evaluation of the effects of deuterium depleted water consumption on 4 patients with brain metastases from lung cancer. Integr. Cancer Ther. 7, 172–181 (2008).
-
Lajos, R. et al. A miRNAs profile evolution of triple negative breast cancer cells in the presence of a possible adjuvant therapy and senescence inducer. Journal of B.U.ON. 23(3), 692–705 (2018).
-
Somlyai, G. et al. Pre-clinical and clinical data confirm the anticancer effect of deuterium depletion. Biomacromol J. 2(1), 1–7 (2016).
-
Yavari, K. & Kooshesh, L. Deuterium depleted water inhibits the proliferation of human MCF7 breast cancer cell lines by inducing cell cycle arrest. Nutr. Cancer. 71, 1019–1029 (2019).
-
Hang, M., Huynh, V. & Meyer, T. J. Colossal kinetic isotope effects in proton-coupled electron transfer. PNAS. 101(36), 13138–13141 (2004).
-
Basov, A. A. et al. Influence of deuterium depleted water on the isotope D/H composition of liver tissue and morphological development of rats at different periods of ontogenesis. Iranian Biomed. J. 23(2), 129–141 (2019).
-
Boros, L. G. et al. Submolecular regulation of cell transformation by deuterium depleting water exchange reactions in the tricarboxylic acid substrate cycle. Med. Hypotheses. 87, 69–74 (2016).
-
Xie, X. & Zubarev, R. A. On the effect of planetary stable isotope compositions on growth and survival of terrestrial organisms. PLoS One. 12(1), e0169296, https://doi.org/10.1371/journal.pone.0169296 (2017).
-
Basov, A., Fedulova, L., Baryshev, M. & Dzhimak, S. Deuterium-depleted water influence on the isotope 2H/1H regulation in body and individual adaptation. Nut. 11, 1903, https://doi.org/10.3390/nu11081903 (2019).
-
Zlatska, A. et al. In vitro study of deuterium effect on biological properties of human cultured adipose-derived stem cells. Sci. World J. 5454367, https://doi.org/10.1155/2018/5454367 (2018).
-
Zlatska, O. V., Zubov, D. O., Vasyliev, R. G., Syroeshkin, A. V. & Zlatskiy, I. A. Deuterium effect on proliferation and clonogenic potential of human dermal fibroblasts in vitro. Probl. Cryobiol Cryomed. 28(1), 049–053 (2018).
-
Kamm, R., Lammerding, J. & Mofrad, M. Cellular nanomechanics (Springer Handbook of Nanotechnology) (Springer Berlin Heidelberg, 2010).
-
Murphy, M. B., Moncivais, K. & Caplan, A. I. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp. Mol. Med. 45, 54, https://doi.org/10.1038/emm.2013.94 (2013).
-
Doorn, J., Moll, G. & Le Blanc, K. Therapeutic applications of mesenchymal stromal cells: paracrine effects and potential improvements. Tissue Eng. 18(2), 101–115, https://doi.org/10.1089/ten.teb.2011.0488 (2012).
-
Caplan, A. I. Why are MSCs therapeutic? New data: new insight. 217, 318–324, https://doi.org/10.1002/path.2469 (2009).
-
Vasyliev, R. G. et al. Comparative analysis of biological properties of large-scale expanded adult neural crest-derived stem cells isolated from human hair follicle and skin dermis. Stem Cells Int. 9640790, https://doi.org/10.1155/2019/9640790 (2019).
-
Bourin, P. et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the international federation for adipose therapeutics and science (IFATS) and the international society for cellular therapy (ISCT). Cytotherapy. 15(6), 641–648, https://doi.org/10.1016/j.jcyt.2013.02.006 (2013).
-
Prockop, D., Phinney, D. & Blundell, B. Mesenchymal stem cells: methods and protocols. Methods Mol. Biol. 449, 192 (2008).
-
O’Brien, J., Wilson, I. & Orton, T. Investigation of the alamar blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 267(17), 5421–5426 (2000).
-
Gimble, J. M. & Guilak, F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 5(5), 362–369, https://doi.org/10.1080/14653240310003026 (2003).
-
Baer, P. & Geiger Adipose-derived mesenchymal stromal/stem cells: tissue localization, characterization, and heterogeneity. Stem Cells Int. 2012, 1–11, https://doi.org/10.1155/2012/812693 (2012).
-
Lobyshev, V. N. & Kalinichenko, L. P. Isotopic effects in biological systems. (ed. Nauka) (Moscow, 1978).
-
Cleland, W. W. The use of isotope effects to determine enzyme mechanisms. JBC. 278(52), 51975–51984 (2003).
-
Lewis, G. N. Biology of heavy water. Science. 79(2042), 151–153 (1934).
-
Harvey, E. N. Biological effects of heavy water. Biol Bull. 66(2), 91–96 (1934).
-
Goncharuk, V. V., Pleteneva, T. V., Uspenskaya, E. V. & Syroeshkin, A. V. Controlled chaos: Heterogeneous catalysis. J. Water Chem. Technol. 39, 325–330 (2017).
-
McCluney, K. E. & Sabo, J. L. Tracing water sources of terrestrial animal populations with stable isotopes: laboratory tests with crickets and spiders. PLoS One. 5, 1–11 (2010).
-
Greene, M. E. et al. Isolation of the human peroxisome proliferator activated receptor gamma cDNA: expression in hematopoietic cells and chromosomal mapping. Gene Expression. 4(4–5), 281–299 (1995).
-
Elbrecht, A. et al. Molecular cloning, expression and characterization of human peroxisome proliferator activated receptors gamma 1 and gamma 2. Biochem. Biophys. Res. Com. 224(2), 431–437, https://doi.org/10.1006/bbrc.1996.1044 (1996).
-
Michalik, L. et al. International union of pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol. Rev. 58(4), 726–741, https://doi.org/10.1124/pr.58.4.5 (2006).
-
Baxa, C. A. et al. Human adipocyte lipid-binding protein: purification of the protein and cloning of its complementary DNA. Biochem. 28(22), 8683–8690, https://doi.org/10.1021/bi00448a003 (1989).
-
Furuhashi, M. et al. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature. 447(7147), 959–965, https://doi.org/10.1038/nature05844 (2007).
-
Shum, B. O. et al. The adipocyte fatty acid-binding protein aP2 is required in allergic airway inflammation. J. Clin. Invest. 116(8), 2183–2192, https://doi.org/10.1172/JCI24767 (2006).
-
Maeda, K. et al. Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes. Cell Metab. 1(2), 107–119, https://doi.org/10.1016/j.cmet.2004.12.008 (2005).
-
Hao, J. et al. Circulating adipose fatty acid binding protein is a new link underlying obesity-associated breast/mammary tumor development. Cell Metab. 28(5), 689–705, https://doi.org/10.1016/j.cmet.2018.07.006 (2018).
-
Hao, J. et al. Expression of adipocyte/macrophage fatty acid-binding protein in tumor-associated macrophages promotes breast cancer progression. Cancer Res. 78(9), 2343–2355, https://doi.org/10.1016/j.cmet.2018.07.006 (2018).
-
Barr, V. A., Malide, D., Zarnowski, M. J., Taylor, S. I. & Cushman, S. W. Insulin stimulates both leptin secretion and production by rat white adipose tissue. Endocrinol. 138(10), 4463–4472 (1997).
-
Ye, F., Than, A., Zhao, Y., Goh, K. H. & Chen, P. Vesicular storage, vesicle trafficking, and secretion of leptin and resistin: the similarities, differences, and interplays. J. Endocrinol. 206(1), 27–36 (2010).
-
Bornstein, S. R. et al. Immunohistochemical and ultrastructural localization of leptin and leptin receptor in human white adipose tissue and differentiating human adipose cells in primary culture. Diabetes. 49(4), 532–538 (2000).
-
Díez, J. J. & Iglesias, P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Europ. J. Endocrinol. 148(3), 293–300, https://doi.org/10.1530/eje.0.1480293 (2003).
-
Ukkola, O. & Santaniemi, M. Adiponectin: a link between excess adiposity and associated comorbidities? J. Mol. Med. 80(11), 696–702, https://doi.org/10.1007/s00109-002-0378-7 (2002).
-
Bauche, I. B. et al. Overexpression of adiponectin targeted to adipose tissue in transgenic mice: impaired adipocyte differentiation. Endocrinol. 148(4), 1539–1549, https://doi.org/10.1210/en.2006-0838 (2007).
-
Yamauchi, T. et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nature Medicine. 7(8), 941–946, https://doi.org/10.1038/90984 (2001).
-
Nedvídková, J., Smitka, K., Kopský, V. & Hainer, V. Adiponectin, an adipocyte-derived protein. Physiological Res. 54(2), 133–140 (2005).
-
Liu, M. & Liu, F. Up- and down-regulation of adiponectin expression and multimerization: mechanisms and therapeutic implication. Biochimie. 94(10), 2126–2130, https://doi.org/10.1016/j.biochi.2012.01.008 (2012).
-
Mead, J. R., Irvine, S. A. & Ramji, D. P. Lipoprotein lipase: structure, function, regulation, and role in disease. J. Mol. Med. 80(12), 753–769, https://doi.org/10.1007/s00109-002-0384-9 (2002).
-
Rinninger, F. et al. Lipoprotein lipase mediates an increase in the selective uptake of high density lipoprotein-associated cholesteryl esters by hepatic cells in culture. J. Lipid Res. 39(7), 1335–1348 (1998).
-
Ma, Y. et al. Mutagenesis in four candidate heparin binding regions (residues 279-282, 291-304, 390-393, and 439-448) and identification of residues affecting heparin binding of human lipoprotein lipase. J. Lipid Res. 35(11), 2049–2059 (1994).
-
Crichton, P. G., Lee, Y. & Kunji, E. R. The molecular features of uncoupling protein 1 support a conventional mitochondrial carrier-like mechanism. Biochimie. 134, 35–50, https://doi.org/10.1016/j.biochi.2016.12.016 (2017).
-
Zhao, L. et al. Specific interaction of the human mitochondrial uncoupling protein 1 with free long-chain fatty acid. Structure. 25(9), 1371–1379.e3, https://doi.org/10.1016/j.str.2017.07.005 (2017).
-
Chathoth, S. et al. Association of uncoupling protein 1 (UCP1) gene polymorphism with obesity: a case-control study. BMC Med Genet. 19(1), 203, https://doi.org/10.1186/s12881-018-0715-5 (2018).
-
Kozak, L. P. & Anunciado-Koza, R. UCP1: its involvement and utility in obesity. Int. J. Obesity. 32(Suppl 7), S32–8, https://doi.org/10.1038/ijo.2008.236 (2008).
-
Basov, A., Fedulova, L., Vasilevskaya, E. & Dzhimak, S. Possible mechanisms of biological effects observed in living systems during 2H/1H isotope fractionation and deuterium interactions with other biogenic isotopes. Molecules. 24, 4101, https://doi.org/10.3390/molecules24224101 (2019).
-
Halenova, T., Zlatskiy, I., Syroeshkin, A., Maximova, T. & Pleteneva, T. Deuterium-depleted water as adjuvant therapeutic agent for treatment of diet-induced obesity in rats. Molecules. 25, 23, https://doi.org/10.3390/molecules25010023 (2020).
Acknowledgements
The publication has been prepared with the support of the “RUDN University Program 5–100”.
Author information
Authors and Affiliations
Contributions
Conceptualization - Alona V. Zlatska, Roman G. Vasyliev, Dmytro O. Zubov, Igor A. Zlatskiy, Anton V. Syroeshkin; Data curation - Alona V. Zlatska, Roman G. Vasyliev, Inna M. Gordiienko, Anzhela E. Rodnichenko, Maria A. Vulf, Dmytro O. Zubov, Larisa S. Litvinova, Igor A. Zlatskiy; Formal analysis - Alona V. Zlatska, Roman G. Vasyliev, Maria A. Vulf, Dmytro O. Zubov, Larisa S. Litvinova, Tatiana V. Grebennikova, Igor A. Zlatskiy, Anton V. Syroeshkin; Investigation - Roman G. Vasyliev, Svitlana N. Novikova, Larisa S. Litvinova, Tatiana V. Grebennikova, Igor A. Zlatskiy, Anton V. Syroeshkin; Methodology - Alona V. Zlatska, Roman G. Vasyliev, Inna M. Gordiienko, Anzhela E. Rodnichenko, Maria A. Morozova, Maria A. Vulf, Dmytro O. Zubov, Svitlana N. Novikova, Larisa S. Litvinova, Tatiana V. Grebennikova, Igor A. Zlatskiy, Anton V. Syroeshkin; Project administration - Roman G. Vasyliev, Dmytro O. Zubov, Svitlana N. Novikova, Larisa S. Litvinova, Tatiana V. Grebennikova, Igor A. Zlatskiy, Anton V. Syroeshkin; Resources - Alona V. Zlatska, Roman G. Vasyliev, Dmytro O. Zubov, Larisa S. Litvinova, Igor A. Zlatskiy, Anton V. Syroeshkin; Software - Alona V. Zlatska, Roman G. Vasyliev, Anzhela E. Rodnichenko, Igor A. Zlatskiy; Supervision - Roman G. Vasyliev, Svitlana N. Novikova, Tatiana V. Grebennikova, Igor A. Zlatskiy, Anton V. Syroeshkin; Validation - Alona V. Zlatska, Roman G. Vasyliev, Dmytro O. Zubov, Larisa S. Litvinova, Tatiana V. Grebennikova, Igor A. Zlatskiy, Anton V. Syroeshkin; Visualization - Alona V. Zlatska, Roman G. Vasyliev, Anzhela E. Rodnichenko, Igor A. Zlatskiy; Writing original draft - Alona V. Zlatska, Roman G. Vasyliev, Inna M. Gordiienko, Maria A. Morozova, Igor A. Zlatskiy, Anton V. Syroeshkin; Writing review & editing - Alona V. Zlatska, Roman G. Vasyliev, Inna M. Gordiienko, Anzhela E. Rodnichenko, Maria A. Morozova, Maria A. Vulf, Dmytro O. Zubov, Svitlana N. Novikova, Larisa S. Litvinova, Tatiana V. Grebennikova, Igor A. Zlatskiy, Anton V. Syroeshkin; All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zlatska, A., Vasyliev, R.G., Gordiienko, I.M. et al. Effect of the deuterium on efficiency and type of adipogenic differentiation of human adipose-derived stem cells in vitro. Sci Rep 10, 5217 (2020). https://doi.org/10.1038/s41598-020-61983-3
-
Received
-
Accepted
-
Published
-
DOIhttps://doi.org/10.1038/s41598-020-61983-3